Altered markers of brain metabolism and excitability are associated with executive functioning in young children exposed to alcohol in utero – PubMed Black Hawk Supplements
BLACK HAWK: Best lions mane supplement for energy
Published article
Prenatal alcohol exposure (PAE) is the leading known cause of birth defects and cognitive disabilities, with impacts on brain development and executive functioning. Abnormalities in structural and functional brain features are well-documented in children with PAE, but the effects of PAE on brain metabolism in children have received less attention. Levels of brain metabolites can be measured non-invasively using magnetic resonance spectroscopy (MRS). Here, we present the first study of…
Black Hawk Supplements, best supplements in the UK

doi: 10.1007/s11011-024-01432-6.
Meaghan V Perdue 1 2 3 , Mohammad Ghasoub 4 5 6 , Madison Long 4 5 6 , Marilena M DeMayo 4 5 6 7 8 , Tiffany K Bell 4 5 6 , Carly A McMorris 5 6 9 , Deborah Dewey 5 6 10 11 , W Ben Gibbard 5 10 , Christina Tortorelli 12 , Ashley D Harris 4 5 6 , Catherine Lebel 4 5 6
Affiliations
- PMID: 39570479
- PMCID: PMC11582302
- DOI: 10.1007/s11011-024-01432-6
Altered markers of brain metabolism and excitability are associated with executive functioning in young children exposed to alcohol in utero
Meaghan V Perdue et al. Metab Brain Dis. .
Abstract
Prenatal alcohol exposure (PAE) is the leading known cause of birth defects and cognitive disabilities, with impacts on brain development and executive functioning. Abnormalities in structural and functional brain features are well-documented in children with PAE, but the effects of PAE on brain metabolism in children have received less attention. Levels of brain metabolites can be measured non-invasively using magnetic resonance spectroscopy (MRS). Here, we present the first study of PAE-related brain metabolite differences in early childhood (ages 3-8 years) and their associations with cognitive performance, including executive functioning (EF) and pre-reading skills. We measured metabolites in two cohorts of children with PAE and unexposed children using MRS in the anterior cingulate cortex (ACC; cohort 1) and left temporo-parietal cortex (LTP; cohort 2). Total choline (tCho), a marker of membrane/myelin metabolism, was elevated in both regions in children with PAE compared to unexposed children, and glutamate + glutamine (Glx), a marker of excitability, was elevated in the ACC. The PAE group exhibited more difficulties with EF, and higher tCho was associated with better EF in both PAE and unexposed groups. In addition, elevated Glx in the ACC was associated with poorer inhibitory control within the PAE group only. LTP metabolites were not significantly associated with pre-reading skills in PAE or unexposed groups. Together, these findings point to altered membrane metabolism and excitability in young children with PAE. These findings provide new insight to potential mechanisms by which PAE disrupts brain development and cognitive functioning in early childhood.
Keywords: Alcohol; Childhood; Cognition; Magnetic Resonance Spectroscopy (MRS); Metabolism.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
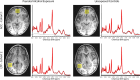
MRS voxel positions and sample spectra. ACC shown on top row, LTP shown on bottom row, participants with PAE shown on left, unexposed participants shown on right. Sample participant age and sex are as follows: Cohort 1 PAE: 4.87 year-old male, Cohort 1 unexposed: 4.89 year-old male, Cohort 2 PAE: 6.14 year-old female, Cohort 2 unexposed: 6.25 year-old female

Metabolite differences by alcohol exposure group shown by box plots indicating group medians and quartiles overlaid on individual data points. Group differences in metabolite levels in the ACC (Cohort 1; left and middle panels) and LTP (Cohort 2; right panel) shown for metabolites with significant effects before FDR correction; only the tCho effect in the ACC remained significant after FDR correction for multiple comparisons (p =.016, pFDR=0.045). Individual data points plotted (purple = unexposed, orange = PAE)
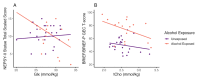
Associations between EF measures and metabolites in ACC (Cohort 1). (A) Inhibitory control was associated with Glx in the ACC (Cohort 1, n = 42) in the PAE group (orange) but not the unexposed group (purple). Inhibitory control indexed by NEPSY-II Statue Total Scaled Score. (B) Associations between PAE status, ACC tCho levels, and EF (Cohort 1, n = 39). Fit lines represent negative association between tCho and EF within each group for unexposed (red) and PAE (yellow). EF indexed by BRIEF/BRIEF-P GEC T-score: higher scores indicate more difficulties in executive functioning
References
-
- Adams JW, Negraes PD, Truong J, Tran T, Szeto RA, Guerra BS, Herai RH, Teodorof-Diedrich C, Spector SA, Del Campo M, Jones KL, Muotri AR, Trujillo CA (2023) Impact of alcohol exposure on neural development and network formation in human cortical organoids. Mol Psychiatry 28(4):1571–1584 – PMC – PubMed
-
- Ashburner J, Friston KJ (2005) Unified segmentation. NeuroImage 26(3):839–851. 10.1016/J.NEUROIMAGE.2005.02.018 – PubMed
-
- Astley SJ, Richards T, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K (2009) Magnetic resonance spectroscopy outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Magn Reson Imaging 27(6):760–778. 10.1016/j.mri.2009.01.003 – PMC – PubMed
-
- Baeshen A, Wyss PO, Henning A, O’Gorman RL, Piccirelli M, Kollias S, Michels L (2020) Test–retest reliability of the Brain metabolites GABA and Glx with JPRESS, PRESS, and MEGA-PRESS MRS sequences in vivo at 3T. J Magn Reson Imaging 51(4):1181–1191. 10.1002/JMRI.26921 – PubMed
MeSH terms
Substances
Grants and funding
LinkOut – more resources
-
Full Text Sources
BLACK HAWK: Best shilajit supplement for women
Read the original publication: