ACAT1 Induces the Differentiation of Glioblastoma Cells by Rewiring Choline Metabolism – PubMed Black Hawk Supplements
BLACK HAWK: Best shilajit supplement for sleep
Published article
Abnormal differentiation of cells is a hallmark of malignancy. Induction of cancer-cell differentiation is emerging as a novel therapeutic strategy with low toxicity in hematological malignances, but whether such treatment can be used in solid tumors is not known. Here, we uncovered a novel function of acetyl coenzyme A acetyltransferase (ACAT1) in regulating the differentiation of glioblastoma (GBM) cells. Inhibition of ACAT1 promoted the differentiation of GBM cells into astrocytes but also…
Black Hawk Supplements, best supplements in the UK

. 2024 Oct 14;20(14):5576-5593.
doi: 10.7150/ijbs.96651. eCollection 2024.
Shen You 1 2 , Ming-Jin Wang 1 , Zhen-Yan Hou 1 3 , Wei-Da Wang 1 , Zhi-Hui Zhang 1 , Ting-Ting Du 1 , Shu-Ying Li 1 , Yi-Chen Liu 1 , Ni-Na Xue 1 , Xiao-Min Hu 4 , Xiao-Guang Chen 1 5 , Ming Ji 1 6
Affiliations
- PMID: 39494339
- PMCID: PMC11528465
- DOI: 10.7150/ijbs.96651
ACAT1 Induces the Differentiation of Glioblastoma Cells by Rewiring Choline Metabolism
Shen You et al. Int J Biol Sci. .
Abstract
Abnormal differentiation of cells is a hallmark of malignancy. Induction of cancer-cell differentiation is emerging as a novel therapeutic strategy with low toxicity in hematological malignances, but whether such treatment can be used in solid tumors is not known. Here, we uncovered a novel function of acetyl coenzyme A acetyltransferase (ACAT1) in regulating the differentiation of glioblastoma (GBM) cells. Inhibition of ACAT1 promoted the differentiation of GBM cells into astrocytes but also delayed tumor growth. Mechanistically, suppression of ACAT1 restored mitochondrial function and led to metabolic “reprogramming” in GBM cells: reduction of fatty-acid oxidation and acetyl-CoA, but an increase in free fatty acids. Importantly, ACAT1 negatively regulated the choline metabolic pathway, which is crucial for the differentiation of GBM cells. Finally, we demonstrated that a naturally available substance, chlorogenic acid (CHA), could inhibit phosphorylation of ACAT1 and so delay GBM progression, CHA is a promising candidate to treat GBM because it could induce the differentiation of cancer cells.
Keywords: acetyl coenzyme A acetyltransferase; cell differentiation; choline; glioblastoma.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
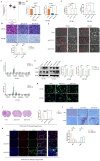
ACAT1 KD in GBM cells leads to differentiation into astrocytes. (A) GEPIA data indicated that ACAT1 had high expression in glioma. (B) ACAT1 expression was knocked down at mRNA levels in U87 MG and U251 MG cells. (C) Flow cytometry was used to determine the percentage of EdU cells proliferating after ACAT1 KD in GBM cells. (D) Representative images of invaded cells after ACAT1 KD in GBM cells. Scale bar, 100 μm. (E) Effect of ACAT1 KD on the morphology of U87 MG and U251 MG cells. Microscopy images were captured. Scale bar, 100 μm. (F) Relative mRNA expression of typical markers in stem cells, astrocytes, oligodendrocytes, and neurons of two ACAT1 KD cell lines were detected by real-time RT-qPCR. (G) Western blots of GFAP and S100b in ACAT1 KD GBM cells. β-actin is used as a control. (H) Immunofluorescence analyses of GFAP expression after ACAT1 KD (green). DAPI is shown in blue. Scale bar, 200 μm. (I) The striatum of Balb/c nude mice was inoculated with U87 MG shACAT1 cells (n = 5/group), and two mice were selected randomly from each group to be killed for H&E staining, scale bar, 1000 μm. Tumor volume was observed by NMR imaging, RadiAntViewer software was used to quantify tumor size. (J) Expression of Ki67 and GFAP was analyzed by immunohistochemistry, scale bar, 100 μm. (K) Expression of Ki67 and GFAP was analyzed by mIHC, scale bar, 20 μm. Region of interest, scale bar, 10 μm.
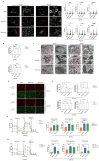
Effect of ACAT1 KD on mitochondrial structure and function in GBM cells. (A) Multi-modal structured light super-resolution microscopy (multi-SIM) was used to observe mitochondrial morphology. Scale bar, 5 μm. Region of interest, scale bar, 2 μm. (B) The red fluorescence of MitoTracker in ACAT1 KD GBM cells was analyzed by flow cytometry. (C) Changes in mitochondrial cristae structures in ACAT1 KD GBM cells under transmission electron microscopy. Scale bar, 1 μm. Region of interest, scale bar, 0.5 μm. (D-E) The mitochondrial membrane potential of GBM cells after ACAT1 KD was observed by flow cytometry and fluorescence microscopy. The mitochondrial membrane potential was determined by JC-1 staining. Red represents JC-1 aggregates; Green represents the JC-1 monomers. Scale bar, 100 μm. (F-G) OCR was monitored in real time using an extracellular flux analyzer. Quantitative analysis of basal respiration, maximum respiration, and ATP production was undertaken.
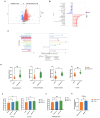
ACAT1 KD activates choline metabolism in GBM cells. (A) Volcano maps comparing differential metabolites in ACAT1 KD GBM cells. (B) Matchstick analysis for the metabolites of ACAT1 KD in GBM cells with upregulated and downregulated expression. (C) Pathway analyses of ACAT1 KD differentially enriched metabolites in GBM cells. (D) Differential metabolites in the choline metabolic pathway. (E) PC levels were examined in U87 MG and U251 MG cells of shACAT1. (F) PC levels were examined in U87 MG orthotopic tumor tissues. (G) Changes in choline levels in the brain of ACAT1-/- and ACAT1 wild type mice.

Activation of the choline metabolic pathway promotes the differentiation of GBM cells. (A) Choline metabolic pathway (schematic). (B) GBM cells were treated with citicoline (10 μM) and PC (0.1 μM), and GFAP was detected by western blotting. (C-D) Overexpression of CHKα and CCTα in GBM cells, and western-blot analysis of changes in GFAP, CHKα, and CCTα in GBM cells. (E-F) Silent expression of CHKα and CCTα in the construct of shACAT1 #2 stably transfected cell lines, and western-blot analysis of changes in GFAP, CHKα, and CCTα. (G) Treatment of GBM cells with an inhibitor of CCTα, miltefosine (10 μM), shACAT1 #2 stably transfected cell line, and western-blot analysis of GFAP changes. (H) Expression of CHKα and CCTα in U87 MG orthotopic tumor tissues was analyzed by immunohistochemistry. Scale bar, 100 μm.
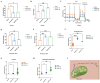
ACAT1 restrained the generation of PC by adjusting FAO. (A-B) AcCoA and FFAs were detected in U87 MG cells of shACAT1. (C) FAO was examined by an extracellular flux analyzer. (D-E) AcCoA and FFAs were detected in orthotopic tumors. (F-H) PS, CDP-DG, and PE were measured by MS. (I) ACAT1 knockdown promoted the PC pathway (schematic).
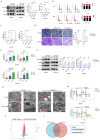
CHA regulated the differentiation of GBM cells by inhibiting p-ACAT1 (Tyr407). (A) GBM cells were treated with CHA (25 μM or 50 μM). After 24 h, western blotting was done to ascertain the change in p-ACAT1 (Tyr407). (B) Cell-cycle arrest occurred in G0/G1 phase in GBM cells treated with CHA (25 μM or 50 μM) for 24 h. (C) The percentage of proliferating EdU-positive cells were measured by flow cytometry, and both cell types were treated with CHA (25 μM or 50 μM) for 7 days. (D) Representative images of invasive cells treated with CHA (25 or 50 μM) for 7 days. Scale bar, 100 μm. (E) Relative mRNA expression of GFAP and β3-tubulin in two cell lines treated with CHA (25 μM or 50 μM) for 24 h were detected by real-time RT-qPCR. (F) Western blots of GFAP, β3-tubulin, and p21 in GBM cells treated with CHA (25 μM or 50 μM) for 24 h. (G) Transmission electron microscopy of GBM cells treated or mot treated with CHA. Scale bar, 1 μm. Region of interest, scale bar, 0.5 μm. (H) OCR assay was determined using GBM cells treated with CHA (25 μM or 50 μM) for 7 days. (I) Volcano maps were used to analyze the differential metabolites of GBM cells treated with CHA (50 μM) for 7 days. (J) Venn diagram representing 27 identical differential metabolites between GBM cells treated with CHA and ACAT1 KD.
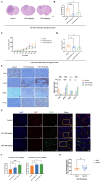
CHA promoted the differentiation of GBM cells in vivo and activated choline metabolism. (A-B) Balb/c nude mice were inoculated with U87 MG cells in the striatum (n =5/group). Three days after inoculation, mice were injected intraperitoneally with vehicle or CHA (20/40 mg/kg/d). We measured tumor size through MRI and killed mice at the endpoint (bodyweight of mice was decreased by 20%) for H&E staining of a brain cross-sections, Scale bar, 1000 μm. (C-D) Balb/c nude mice were inoculated subcutaneously with U251 MG cells (n = 6/group). Mice were injected intraperitoneally with CHA (20/40 mg/kg/d). Bodyweight and tumor volume (mean ± SD) were recorded every 3 days for subcutaneous inoculation. (E) Immunohistochemical analyses for the expression of Ki67, GFAP, CHKα and CCTα. Scale bar, 100 μm. (F) Expression of Ki67 and GFAP was analyzed by mIHC, scale bar, 20 μm. Region of interest, scale bar, 10 μm. (G) PC levels were determined in tumor tissues treated with CHA (20/40 mg/kg, i.p.). (H) Patients with glioma were treated with CHA for 30 days and their serum were used to determine changes in PC levels. Data are the mean ± SD (n = 17 per group).
Similar articles
-
From mitochondria to tumor suppression: ACAT1’s crucial role in gastric cancer.
He W, Li Y, Liu SB, Chang Y, Han S, Han X, Ma Z, Amin HM, Song YH, Zhou J. He W, et al. Front Immunol. 2024 Aug 23;15:1449525. doi: 10.3389/fimmu.2024.1449525. eCollection 2024. Front Immunol. 2024. PMID: 39247186 Free PMC article.
-
Tetrameric Acetyl-CoA Acetyltransferase 1 Is Important for Tumor Growth.
Fan J, Lin R, Xia S, Chen D, Elf SE, Liu S, Pan Y, Xu H, Qian Z, Wang M, Shan C, Zhou L, Lei QY, Li Y, Mao H, Lee BH, Sudderth J, DeBerardinis RJ, Zhang G, Owonikoko T, Gaddh M, Arellano ML, Khoury HJ, Khuri FR, Kang S, Doetsch PW, Lonial S, Boggon TJ, Curran WJ, Chen J. Fan J, et al. Mol Cell. 2016 Dec 1;64(5):859-874. doi: 10.1016/j.molcel.2016.10.014. Epub 2016 Nov 17. Mol Cell. 2016. PMID: 27867011 Free PMC article.
-
The recent insights into the function of ACAT1: A possible anti-cancer therapeutic target.
Goudarzi A. Goudarzi A. Life Sci. 2019 Sep 1;232:116592. doi: 10.1016/j.lfs.2019.116592. Epub 2019 Jun 19. Life Sci. 2019. PMID: 31228515 Review.
-
Wang M, Wang W, You S, Hou Z, Ji M, Xue N, Du T, Chen X, Jin J. Wang M, et al. Acta Pharm Sin B. 2023 Dec;13(12):4733-4747. doi: 10.1016/j.apsb.2023.09.005. Epub 2023 Sep 15. Acta Pharm Sin B. 2023. PMID: 38045043 Free PMC article.
-
ACAT1/SOAT1 as a therapeutic target for Alzheimer’s disease.
Shibuya Y, Chang CC, Chang TY. Shibuya Y, et al. Future Med Chem. 2015;7(18):2451-67. doi: 10.4155/fmc.15.161. Epub 2015 Dec 15. Future Med Chem. 2015. PMID: 26669800 Free PMC article. Review.
References
MeSH terms
Substances
BLACK HAWK: Best ashwagandha supplement for men
Read the original publication:
ACAT1 Induces the Differentiation of Glioblastoma Cells by Rewiring Choline Metabolism – PubMed