Ashwagandha-Induced Hepatic Injury: A Case Report – PubMed Black Hawk Supplements
BLACK HAWK: High quality ashwagandha supplement for sleep
Published article
A 22-year-old healthy Libyan female suffered moderate to severe liver injury following the ingestion of ashwagandha capsules. Following a latency period of 30 hours, she developed severe itching, fatigue, nausea, and jaundice. Laboratory results showed significantly increased liver enzymes (especially alanine aminotransferase (ALT)) and bilirubin (mainly conjugated bilirubin), along with a slight elevation of alkaline phosphatase (ALP). We used the Roussel Uclaf Causality Assessment Method…
Black Hawk Supplements, best supplements in the UK

Case Reports
. 2024 Oct 15;16(10):e71576.
doi: 10.7759/cureus.71576. eCollection 2024 Oct.
Affiliations
- PMID: 39553154
- PMCID: PMC11564894
- DOI: 10.7759/cureus.71576
Case Reports
Ashwagandha-Induced Hepatic Injury: A Case Report
Fakhruddin Almuzghi et al. Cureus. .
Abstract
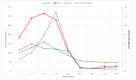
A 22-year-old healthy Libyan female suffered moderate to severe liver injury following the ingestion of ashwagandha capsules. Following a latency period of 30 hours, she developed severe itching, fatigue, nausea, and jaundice. Laboratory results showed significantly increased liver enzymes (especially alanine aminotransferase (ALT)) and bilirubin (mainly conjugated bilirubin), along with a slight elevation of alkaline phosphatase (ALP). We used the Roussel Uclaf Causality Assessment Method (RUCAM) worksheet to link liver injury to ashwagandha. The R ratio was 5.4, and the overall RUCAM score was seven. The peaks of ALT, total bilirubin, and ALP were 315 IU/L, 12.85 mg/dl, and 150 IU/L, respectively. The washout period was 60 days. Symptoms were relieved by ursodeoxycholic acid (UDSA). The clinical assessment and workup for viral hepatitis and autoimmune hepatitis were negative. In conclusion, ashwagandha can cause prolonged hepatocellular liver injury in healthy and young individuals.
Keywords: ashwagandha (withania somnifera); clinical case report; drug-induced liver injury (dili); jaundice; libya.
Copyright © 2024, Almuzghi et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2019. Ashwagandha.
-
- ACG clinical guideline: diagnosis and management of idiosyncratic drug-induced liver injury. Chalasani NP, Maddur H, Russo MW, Wong RJ, Reddy KR. Am J Gastroenterol. 2021;116:878–898. – PubMed
Publication types
BLACK HAWK: Best ashwagandha supplement for mums
Read the original publication: