Intracalvariosseous administration of donepezil microspheres protects against cognitive impairment by virtue of long-lasting brain exposure in mice – PubMed Black Hawk Supplements
BLACK HAWK: Best lions mane supplement for sexual health
Published article
Rationale: Recent studies have demonstrated the direct connections between the skull bone marrow, meninges, and brain. In an effort to explore these connections for the purpose of brain drug delivery, we previously proposed the direct application of CNS drugs into the diploic space between the outer and inner cortex of the skull, namely, intracalvariosseous administration (ICO). It was successfully demonstrated that small molecular to large colloidal drugs can readily reach the brain after ICO…
Black Hawk Supplements, best supplements in the UK

Intracalvariosseous administration of donepezil microspheres protects against cognitive impairment by virtue of long-lasting brain exposure in mice
Ji Hee Kang et al. Theranostics. .
Abstract
Rationale: Recent studies have demonstrated the direct connections between the skull bone marrow, meninges, and brain. In an effort to explore these connections for the purpose of brain drug delivery, we previously proposed the direct application of CNS drugs into the diploic space between the outer and inner cortex of the skull, namely, intracalvariosseous administration (ICO). It was successfully demonstrated that small molecular to large colloidal drugs can readily reach the brain after ICO in mice and rabbits. Here, we report that a single ICO of donepezil microspheres protects cognitive impairment in Alzheimer mouse models over a month-long period. Methods: Donepezil-loaded long-acting microspheres (DPZ@LAM) were prepared with biodegradable poly(DL-lactide-co-glycolide) (PLGA). Pharmacokinetic study and behavioral test were performed to determine the brain exposure and therapeutic effects after ICO of DPZ@LAM in scopolamine-induced memory-deficient mice. Results: DPZ@LAM were capable of a month-long and precisely controlled drug release. After a single ICO of DPZ@LAM, DPZ concentration in brain sustained above the effective therapeutic levels for four weeks. The long-lasting brain exposure also led to significantly recovered cognitive function in scopolamine-induced memory-deficient mice, along with decreased acetylcholinesterase activity and increased brain-derived neurotrophic factor. Conclusions: ICO allows for BBB-bypassing brain drug delivery through the direct connection between the skull bone marrow and brain, providing an alternative approach for the treatment of neurodegenerative diseases with otherwise BBB impermeable CNS drugs.
Keywords: Alzheimer’s disease; BBB-bypassing route; Long-acting injectable PLGA microspheres; brain drug delivery; intraclavariosseous administration.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
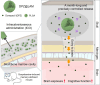
Schematic illustration of protecting against cognitive impairment after ICO of donepezil-loaded long-acting PLGA microspheres (DPZ@LAM) by long-lasting brain exposure in scopolamine-induced memory-deficient mouse models.
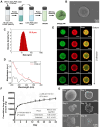
Preparation and characterization of DPZ@LAM. (A) Schematic representation of DPZ@LAM by double emulsion-solvent method. (B) SEM images of DPZ@LAM. (C) Particle size distribution of DPZ@LAM. (D) UV/Vis absorbance spectrum of free DPZ and DPZ@LAM showing four characteristic absorption peak (*) of DPZ. (E) Confocal microscope z-stack projections of a PLGA microsphere showing the distribution of FITC (log P = 4.5, green) and DiD (log P = ~20, red) dyes. Scale bars = 20 µm. In vitro release kinetics and PLGA microspheres erosion analysis. (F) Cumulative release of DPZ over 28 days from DPZ@LAM microsphere of 1 mg. Drug release study was performed under sink conditions in 10 mM PBS pH 7.4 at 37°C with 50 rpm (n = 4). (G) SEM images of a PLGA microsphere depending on the time of storage in PBS 7.4 solution at 37°C.
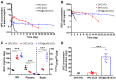
Brain exposure of DPZ after ICO of DPZ@LAM for four weeks in mice as compared to those after ICO and PO of free DPZ. The concentration-time profiles of DPZ in the (A) plasma and (B) brain ISF. (C) Brain to plasma AUC ratio (AUCISF/AUCplasma). (D) DPZ concentration in whole brain (Cbr) at day 28. Statistical significance was indicated by ***p < 0.001.
Biodistribution of DPZ in the plasma, skull, dura and brain after treatment. To investigate distribution of DPZ in different brain regions following ICO of free DPZ and DPZ@LAM, the brain distributions were analyzed in dissected cortex, hippocampus and subcortex. (A) Schematic demonstrating regions of interest selection, including skull, dura and different brain regions, for assessment of DPZ distribution. Biodistribution of DPZ in the plasma, skull, dura and brain at peak time in the ISF profiles after (B) PO of free DPZ, (C) ICO of free DPZ and (D) ICO of DPZ@LAM. Biodistribution of DPZ in the plasma, skull, dura and brain at day 28 after ICO of (E) free DPZ and (F) DPZ@LAM. Orange and aqua bars represent the contralateral (left) and ipsilateral (right) sites, respectively. Statistical significance was indicated by ***p < 0.001.
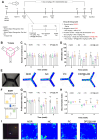
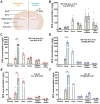
Long-lasting cognitive improvement effect after a single ICO of DPZ@LAM in scopolamine (Sco)-induced memory deficient mice. The mice were intraperitoneally single administered with scopolamine (2 mg/kg) in each test day except normal control (NOR) group. The positive control group (PC) and test group (DPZ@LAM) were treated with DPZ by PO and DPZ@LAM by ICO at a dose of 5 mg/kg as DPZ, respectively. (A) Time schedule of the experimental and treatment designs of behavior test. (B) Schematic diagram of Y-maze test. (C) The number of arm entries on the Y-maze test. (D) Alternation of 5 min session on the Y-maze test. (E) Representative photograph of the Y-maze test and trajectory heat map images of mouse positions in the experimental arena. (F) Schematic diagram of NORT. (G) Preferential index and (H) discrimination index of 3 min session on the NORT. (I) Representative photograph of the NORT and trajectory heat map images of mouse positions in the experimental arena. The data were represented as mean ± SEM (n = 6 – 7 per group) compared with the negative control (NC) group. The differences among the multiple groups were considered by a two-way ANOVA with significant *p < 0.05, **p < 0.01, and ***p < 0.001.

(A) Representative photomicrographs of immunohistochemistry in hippocampal tissue of mice in each group after behavior test by hematoxylin and eosin (H&E) and brain-derived neurotrophic factor (BDNF) staining. The scale bars indicate 200 μm. (B) The BDNF positive (BDNF+) percent area in the hippocampus of each group. (C) Western blot results showing the expression level of BDNF in the brain homogenates of each group on week 3; the β-actin was used as an internal reference. (D) Relative levels of BDNF by quantification of western blot results. (E) Acetylcholine esterase activity test in brain tissue homogenates after behavior test. (mean ± SEM, n = 3). The data were represented as mean ± SEM (n = 3 per group) compared with the negative control (NC) group. The differences among the multiple groups were considered by a one-way ANOVA with significant *p < 0.05, **p < 0.01, and ***p < 0.001.
Similar articles
-
Salem HF, Aboud HM, Abdellatif MM, Abou-Taleb HA. Salem HF, et al. J Pharm Sci. 2024 Jul;113(7):1934-1945. doi: 10.1016/j.xphs.2024.02.014. Epub 2024 Feb 16. J Pharm Sci. 2024. PMID: 38369023
-
Kang JH, Ko YT. Kang JH, et al. Bioeng Transl Med. 2022 Oct 21;8(2):e10424. doi: 10.1002/btm2.10424. eCollection 2023 Mar. Bioeng Transl Med. 2022. PMID: 36925676 Free PMC article.
-
Zhao J, Ren T, Yang M, Zhang Y, Wang Q, Zuo Z. Zhao J, et al. Xenobiotica. 2020 Apr;50(4):389-400. doi: 10.1080/00498254.2019.1643514. Epub 2019 Aug 1. Xenobiotica. 2020. PMID: 31298070
-
[Pharmacological properties of donepezil hydrochloride (Aricept), a drug for Alzheimer’s disease].
Ogura H, Kosasa T, Araki S, Yamanishi Y. Ogura H, et al. Nihon Yakurigaku Zasshi. 2000 Jan;115(1):45-51. doi: 10.1254/fpj.115.45. Nihon Yakurigaku Zasshi. 2000. PMID: 10876815 Review. Japanese.
-
An Update on the Routes for the Delivery of Donepezil.
Zhao ZQ, Chen BZ, Zhang XP, Zheng H, Guo XD. Zhao ZQ, et al. Mol Pharm. 2021 Jul 5;18(7):2482-2494. doi: 10.1021/acs.molpharmaceut.1c00290. Epub 2021 Jun 8. Mol Pharm. 2021. PMID: 34100291 Review.
References
-
- Nordberg A. Dementia in 2014. Towards early diagnosis in Alzheimer disease. Nat Rev Neurol. 2015;11:69–70. – PubMed
-
- Waldemar G. Donepezil in the treatment of patients with Alzheimer’s disease. Expert Rev Neurother. 2001;1:11–9. – PubMed
-
- Matsuoka K, Hirata K, Kokubo N, Maeda T, Tagai K, Endo H. et al. Investigating neural dysfunction with abnormal protein deposition in Alzheimer’s disease through magnetic resonance spectroscopic imaging, plasma biomarkers, and positron emission tomography. Neuroimage Clin. 2024;41:103560. – PMC – PubMed
-
- 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18:700–789. – PubMed
-
- WHO. Fact sheets: Dementia. 2023; Available from: https://www.who.int/news-room/fact-sheets/detail/dementia.
MeSH terms
Substances
BLACK HAWK: Best shilajit supplement for mums
Read the original publication: