Molecular Insights into the Inhibition of Lipid Accumulation in Hepatocytes by Unique Extracts of Ashwagandha – PubMed Black Hawk Supplements
BLACK HAWK: Best shilajit supplement for depression
Published article
We investigated the effect of purified withanolides and extracts derived from Ashwagandha on steatosis, the abnormal accumulation of fat that can lead to non-alcoholic fatty liver disease (NAFLD). Collaborator of ARF (CARF, also known as CDKN2AIP, a protein that regulates hepatic lipid metabolism, fat buildup, and liver damage) was used as an indicator. Six withanolides (Withaferin A, Withanone, Withanolide B, Withanoside IV, Withanoside V, and Withanostraminolide-12 deoxy) reversed the decrease…
Black Hawk Supplements, best supplements in the UK

Molecular Insights into the Inhibition of Lipid Accumulation in Hepatocytes by Unique Extracts of Ashwagandha
Dongyang Li et al. Int J Mol Sci. .
Abstract
We investigated the effect of purified withanolides and extracts derived from Ashwagandha on steatosis, the abnormal accumulation of fat that can lead to non-alcoholic fatty liver disease (NAFLD). Collaborator of ARF (CARF, also known as CDKN2AIP, a protein that regulates hepatic lipid metabolism, fat buildup, and liver damage) was used as an indicator. Six withanolides (Withaferin A, Withanone, Withanolide B, Withanoside IV, Withanoside V, and Withanostraminolide-12 deoxy) reversed the decrease in CARF caused by exposure to free fatty acids (FFAs) in liver-derived cells (HepG2 hepatocytes). After analyzing the effects of these withanolides on CARF mRNA and protein levels, FFA accumulation, protein aggregation, and oxidative and DNA damage stresses, we selected Withaferin A and Withanone for molecular analyses. Using the palmitic-acid-induced fatty acid accumulation stress model in Huh7 cells, we found a significant reduction in the activity of the key regulators of lipogenesis pathways, including sterol regulatory element-binding protein-1c (SREBP-1c), fatty acid synthase (FASN), and peroxisome proliferator-activated receptors (PPARγ and PPARα). This in vitro study suggests that low, non-toxic doses of Withaferin A, Withanone, or Ashwagandha extracts containing these withanolides possess anti-steatosis and antioxidative-stress properties. Further in vivo and clinical studies are required to investigate the therapeutic potential of these Ashwagandha-derived bioactive ingredients for NAFLD.
Keywords: Ashwagandha; CARF expression; lipogenesis; regulation; steatosis; stress; treatment; withanolides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
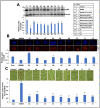
Protective effects of Ashwagandha withanolides and extracts on collaborator ARF CARF expression and lipid accumulation in free fatty acid (FFA)-treated HepG2 cells. (A) Western blot analysis showing a decrease in CARF expression in HepG2 cells treated with FFAs and its reversal in cells co-treated with Ashwagandha withanolides and extracts. β-actin was used as an internal loading control. Quantitation from three independent experiments is shown below. (B) Immunostaining of CARF showing a remarkable decrease in CARF in cells treated with FFAs and its reversal in cells co-treated with Ashwagandha withanolides and extracts. Quantitation from three independent experiments is shown below. (C) Oil Red O staining showing an increase in lipid accumulation in FFA-treated HepG2 cells and its reversal in cells co-treated with Ashwagandha withanolides and extracts. Quantification from three independent experiments is shown below. The quantification graph data in (A–C) were normalized against the FFA group and plotted as fold differences (mean ± SD; n = 3). * p < 0.05, ** p < 0.01, and *** p < 0.001 denote the statistical significance compared with the FFA group (derived from an unpaired Student’s t-test). (B,C) images were captured under a magnification of 20×.
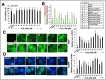
Protective effects of Ashwagandha withanolides and extracts on H2O2-induced oxidative stress in HepG2 cells. (A) Cell viability assay indicating that Ashwagandha withanolides and extracts protected HepG2 cells against a decrease in viability caused by a 400 μM H2O2 treatment. (B) JC-1 fluorescence intensity, plotted as a ratio of monomers (green, indicative of depolarized membrane potential) to aggregates (red, indicative of intact mitochondrial membrane potential), showing that Ashwagandha withanolides and extracts protected HepG2 cells from H2O2-induced membrane depolarization. (C) Representative images of intracellular reactive oxygen species (ROS) levels in HepG2 cells under oxidative stress induced by H2O2, demonstrating the protective effects of some withanolides and Ashwagandha extracts. Quantitation from three independent experiments is shown on the right. (D) Immunostaining of γH2AX foci indicating the protective effects of some withanolides and Ashwagandha extracts on DNA damage in HepG2 cells treated with H2O2. Quantification from three independent experiments is shown on the right. For panels (A,B) and the quantification graphs in (C,D), data were normalized against the stress group and plotted as fold differences. The statistical significance of the data (mean ± SD; n = 3; and * p < 0.05, ** p < 0.01, and *** p < 0.001) was calculated using an unpaired Student’s t-test.
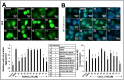
Effects of Ashwagandha withanolides and extracts on protein aggregation and hypoxia stress. (A) GFP-expressing HepG2 cells treated with NaAsO2 showed aggregated/increased GFP intensity. Cells treated with Ashwagandha withanolides and extracts exhibited a reduction in GFP aggregates. Quantification from three independent experiments is shown below. (B) Immunostaining for HIF-1α in HepG2 cells treated with CoCl2, showing an increase. Cells treated with Ashwagandha withanolides and extracts showed a reduction in HIF-1α. Quantitation from three independent experiments is shown below. For the quantitation graphs in (A,B), data were normalized against the stressed groups and plotted as fold differences. The statistical significance of the data (mean ± SD; n = 3; and ** p < 0.01, and *** p < 0.001) was calculated using an unpaired Student’s t-test.
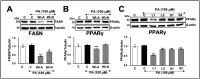
Effects of withanolides and Ashwagandha extracts on key regulators of lipid metabolism. (A) Western blot showing the effect of Withaferin A (Wi-A) and Withanone (Wi-N) on fatty acid synthase (FASN) protein level in Huh7 cells treated with PA. Quantitation from three independent experiments is shown below. (B) Western blot displaying the effect of Wi-A and Wi-N on peroxisome proliferator-activated receptor (PPAR)γ protein levels in palmitic acid (PA)-treated Huh7 cells. Quantitation from three independent experiments is shown below. (C) Western blot analysis showing the effect of Ashwagandha extracts on PPARγ protein levels in Huh7 cells treated with PA. Quantitation from three independent experiments is shown below. For the quantitation graphs, data were normalized against the PA group and plotted as fold differences (mean ± SD; n = 3). The statistical significance of the data (mean ± SD; n = 3; and * p < 0.05, ** p < 0.01, and *** p < 0.001) was calculated using an unpaired Student’s t-test.
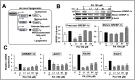
Effects of Ashwagandha extracts on the de novo lipogenesis pathway. (A) A schematic representation of the de novo lipogenesis pathway, key enzymes, and steps involved in fatty acid synthesis is shown. (B) Western blot showing the effects of Ashwagandha extracts on precursor and mature sterol regulatory element-binding protein-1c (SREBP-1c) expression levels in Huh7 cells treated with PA. β-actin was used as a loading control. Quantitation from three independent experiments is shown below. (C) Ashwagandha extracts decreased SREBP-1c mRNA and its target genes Acetyl-CoA Carboxylase 1 (ACC1), fatty acid synthase (FASN) and stearoyl-CoA desaturase 1 (SCD1) in PA-treated HepG2 cells. Data were normalized against the PA group for all panels and plotted as fold differences (mean ± SD; n = 3). The statistical significance of the data (mean ± SD; n = 3; and * p < 0.05, ** p < 0.01, and *** p < 0.001) was calculated using an unpaired Student’s t-test.
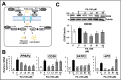
Effects of Ashwagandha extracts on fatty acid uptake and lipogenesis pathway. (A) Schematic diagram of fatty acid uptake and lipogenesis controlled by peroxisome proliferator-activated receptor (PPAR)γ, highlighting the key enzymes and transport proteins involved in these metabolic pathways. (B) Ashwagandha extracts decreased PPARγ mRNA and its target genes cluster of differentiation 36 (CD36), fatty acid transport protein 2 (FATP2), and fatty-acid-binding protein 4 (αP2) in palmitic acid (PA)-treated Huh7 cells. (C) Western blot showing a decrease in CD36 protein in Huh7 cells treated with PA and Ashwagandha extracts compared with PA alone. Quantitation from three independent experiments is shown below. For the quantitation, data were normalized against the PA group and plotted as fold differences (mean ± SD; n = 3). The statistical significance of the data (mean ± SD; n = 3; and * p < 0.05, ** p < 0.01, and *** p < 0.001) was calculated using an unpaired Student’s t-test.
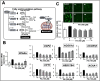
Effects of Ashwagandha extracts on fatty acid β-oxidation and reactive oxygen species (ROS) accumulation. (A) Schematic representation of the fatty acid β-oxidation pathway, highlighting key enzymes and regulatory proteins involved in this metabolic process. (B) Ashwagandha extracts decreased peroxisome proliferator-activated receptor (PPAR)α mRNA and its target genes acyl-CoA oxidase 1 (ACOX1), carnitine palmitoyltransferase 1 (CPT1), 17β-hydroxysteroid dehydrogenase type IV (HSD17B4), and acetyl-coenzyme A acyltransferase 1 (ACAA1) in Huh7 cells treated with both PA and Ashwagandha extracts compared with the PA treatment alone. (C) Cells treated with PA showed an increase in ROS. Ashwagandha extracts decreased the PA-induced ROS level. Quantitation from three independent experiments is shown below. For the quantitation graphs, data were normalized against the PA group and plotted as fold differences (mean ± SD; n = 3). The statistical significance of the data (mean ± SD; n = 3; and * p < 0.05, ** p < 0.01, and *** p < 0.001) was calculated using an unpaired Student’s t-test. The images in C were captured under a magnification of 20×.
References
-
- Kaul S.C., Wadhwa R. Science of Ashwagandha: Preventive and Therapeutic Potentials. Springer; Cham, Switzerland: 2017.
-
- Cavaleri F., Chattopadhyay S., Palsule V., Kar P.K., Chatterjee R. Study of Drug Targets Associated With Oncogenesis and Cancer Cell Survival and the Therapeutic Activity of Engineered Ashwagandha Extract Having Differential Withanolide Constitutions. Integr. Cancer Ther. 2024;23:15347354231223499. doi: 10.1177/15347354231223499. – DOI – PMC – PubMed
MeSH terms
Substances
Grants and funding
The study is supported by grants from AIST (Japan).
BLACK HAWK: Best shilajit supplement for men
Read the original publication: