Shilajit mitigates chemotherapeutic drug-induced testicular toxicity: Study on testicular germ cell dynamics, steroidogenesis modulation, and Nrf-2/Keap-1 signaling – PubMed Black Hawk Supplements
BLACK HAWK: High quality ashwagandha supplement for immune system
Published article
CONCLUSION: The findings underscore the potent androgenic and antioxidant characteristics of Shilajit, as well as its ability to enhance fertility in cases of testicular damage caused by chemotherapeutic drugs.
Black Hawk Supplements, best supplements in the UK

Shilajit mitigates chemotherapeutic drug-induced testicular toxicity: Study on testicular germ cell dynamics, steroidogenesis modulation, and Nrf-2/Keap-1 signaling
Arti Rajpoot et al. J Ayurveda Integr Med. 2024 Jul-Aug.
Abstract
Background: Medications, including chemotherapeutic drugs, contribute to male infertility as external factors by inducing oxidative stress in testicular cells. Shilajit is a naturally occurring bioactive antioxidant used in Ayurvedic medicine to treat a variety of ailments.
Objective: This study examines the potential of Shilajit to counteract the negative effects of the chemotherapeutic drug cyclophosphamide (CPA) on testicular germ cell dynamics.
Material and methods: Male Parkes mice received single intraperitoneal CPA injection (200 mg/kg BW) on day one, followed by daily supplementation of Shilajit (100 and 200 mg/kg BW) for one spermatogenic cycle.
Results: CPA adversely affected testicular germ cell dynamics by inhibiting the conversion of spermatogonia-to-spermatids, altering testicular histoarchitecture, impairing Sertoli cell function and testicular steroidogenesis, and disturbing the testicular oxido-apoptotic balance. Shilajit supplementation restores testicular germ cell dynamics in CPA-exposed mice, as evidenced by improved histoarchitecture of the testis. Shilajit improves testicular daily production and sperm quality by promoting the conversion of spermatogonia (2C) into spermatids (1C), stimulating germ cell proliferation (PCNA), improving Sertoli cell function (N-Cadherin and β-Catenin), and maintaining the Bax/Bcl2 ratio. Additionally, Shilajit enhances testosterone biosynthesis by activating enzymes like 3β-HSD, and 17β-HSD. Shilajit also reduces testicular oxidative stress by increasing antioxidant enzyme activity (SOD) and decreasing lipid peroxidation (LPO). These effects are mediated by upregulation of the antioxidant protein Nrf-2 and downregulation of Keap-1.
Conclusion: The findings underscore the potent androgenic and antioxidant characteristics of Shilajit, as well as its ability to enhance fertility in cases of testicular damage caused by chemotherapeutic drugs.
Keywords: Cyclophosphamide; Oxidative stress; Sertoli cell function; Shilajit; Steroidogenesis; Testicular germ cell dynamics.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
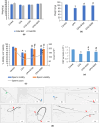
Effect of Shilajit on cyclophosphamide (CPA)-exposed mice; body weight (a), absolute testis weight (b). Effect of Shilajit on sperm count, motility and viability of cauda epididymal spermatozoa of CPA-exposed mice (c). Effect of Shilajit on morphology of cauda epididymal spermatozoa of CPA-exposed mice (d). Note a normal appearance of spermatozoa (green arrow) in control mice (i) and spermatozoa from mice exposed to CPA (ii-ix). (ii & iii) head abnormalities (blue arrow): (ii) spermatozoa with amorphous head; (iii) spermatozoa with isolated head. (iv-ix) tail abnormalities (red arrow): (iv and v) spermatozoa with folded and coiled tail in proximal region, respectively; (vi) spermatozoa with coiled tail in medial region; (vii and viii) spermatozoa with coiled and folded tail in distal region respectively; (ix) spermatozoa with headless tail. Effect of Shilajit on testicular daily sperm production of CPA-exposed mice (e). * and # indicated significant different from controls (p 0.05) and CPA-exposed mice (p 0.05) respectively. BW: Body weight.
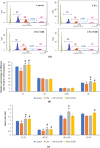
A representative histogram demonstrating the distribution of DNA content in testicular germ cells in mice exposed to various treatments (a). The effect of different treatments on the distribution of different types of germ cells in testis (b) and the ratio of germ cells (c). Values are mean ± SEM. S-Ph: S-Phase. *significant difference from controls, # significant difference from CPA-exposed mice, (p < 0.05).
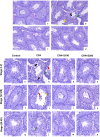
Photomicrograph of seminiferous tubules of mouse testis (PAS-haematoxylin staining) (a-d; stage-specific photomicrograph e-p). Photomicrograph of testis of control mice showing normal morphological features of seminiferous tubules (a and e-g). Photomicrograph of testis of CPA exposed mice shows atrophic appearances of seminiferous tubules (b and h-j). Note the appearance of reduction of germinal epithelium height (yellow arrow), vacuolization (green arrow), germinal epithelium loosening (black arrow), and exfoliated germ cells (red arrow). However, 100 mg/kg (c and k-m) and 200 mg/kg (d and n-p) Shilajit treated CPA-exposed mice testis, showing restoration of normal histoarchitecture in seminiferous tubules. 200× (Scale bar = 207.5 μm); 400× (Scale bar = 103.8 μm).
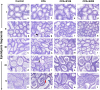
Photomicrograph of mouse epididymis through segment I–V (PAS-haematoxylin staining). Control mice showing normal features (a–e). CPA-exposed mice showing vacuole-like space (black arrow) in epididymal epithelium, exfoliated germ cells (red arrow) and very less numbers of spermatozoa (blue arrow) in lumen of segment V (f–j). Shilajit treatment after CPA exposure shows a dose-dependent increase in the number of spermatozoa in lumen of segment V of the epididymis (k-o; p-t). 200× (Scale bar = 207.5 μm).
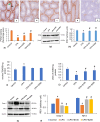
Immunolocalization of PCNA in different treatment groups. PCNA-positive cells in control mice are denoted by black and green arrows in spermatogonia and spermatocytes respectively. PCNA-immunostaining is confined nearly to spermatogonia in CPA-exposed mice (b) compared to controls (a). However, 100 and 200 mg/kg Shilajit (c and d respectively) administration results in a dose-dependent increase in PCNA-immunostaining when compared to mice that were exposed to CPA. A photomicrograph of negative control has been provided (e); 200× (Scale bar = 207.5 μm). Semi-quantification of PCNA immunostaining showed a significant decrease in CPA-exposed mice compared to controls and Shilajit significantly increased the PCNA immunostaining in the testis compared with CPA-exposed mice (f). Western blot analysis of PCNA in the testes of different treatment groups (g). The expression levels of PCNA have been normalized against its corresponding β-actin loading controls. Densitometric data of PCNA of triplicate blot are represented as mean of IRDV ± SEM (h). Effect of Shilajit on lipid peroxidation (i) and activity of Superoxide dismutase (j) of CPA exposed mice. Western blot analyses of Keap-1 and Nrf-2 in the testes of different treated groups (k). The expression levels of Keap-1 and Nrf-2 have been normalized against their corresponding β-actin loading controls. Densitometric data of Keap-1 and Nrf-2 of triplicate blot are presented as mean IRDV ± SEM (l). *significant difference from controls, # significant difference from CPA-exposed mice, (p < 0.05). TBARS: thiobarbituric acid‐reactive substances; Keap-1: Kelch Like ECH Associated Protein 1; Nrf-2: NF-E2–related factor 2.
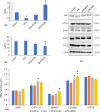
Effect of Shilajit on serum testosterone (a) and serum estradiol level (b) of CPA-exposed mice. Western blot analyses of StAR, CYP11A1, 3β-HSD, 17β-HSD & CYP-19 in the testes of different treatment groups (c). The expression levels of each protein have been normalized against its corresponding β-actin loading control. Densitometric data from a triplicate blot of StAR, CYP11A1, 3β-HSD, 17β-HSD & and CYP-19 are presented as mean of IRDV ± SEM (d). * significant difference from controls, # significant difference from CPA-exposed mice, (p < 0.05). StAR: steroidogenic acute regulatory (protein); HSD: hydroxysteroid dehydrogenases.
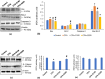
Effect of Shilajit on apoptotic markers of different treated groups (a and b). Western blot analyses of Bax, Bcl-2, and caspase‐3 in the testis of different treated groups (a). The expression levels of each protein have been normalized against its corresponding β-actin loading control. Densitometric data from a triplicate blot of Bax, Bcl-2, and caspase-3 are presented as the mean of IRDV ± SEM (b). Effect of Shilajit on Sertoli cell markers of different treated groups (c–e). Western blot analyses of N-cadherin and β-catenin in the testes of different treated groups (c). The expression levels of each protein have been normalized against its corresponding β-actin loading control. Densitometric data from a triplicate blot of N-cadherin and β-catenin are presented as mean of IRDV ± SEM (d and e respectively). *significant difference from controls, # significant difference from CPA-exposed mice, (p < 0.05).
Similar articles
-
Profertility effects of Shilajit on cadmium-induced infertility in male mice.
Mishra RK, Jain A, Singh SK. Mishra RK, et al. Andrologia. 2018 Oct;50(8):e13064. doi: 10.1111/and.13064. Epub 2018 Jun 27. Andrologia. 2018. PMID: 29947420
-
Yadav A, Yadav K, Rajpoot A, Lal B, Mishra RK. Yadav A, et al. Andrologia. 2022 Dec;54(11):e14575. doi: 10.1111/and.14575. Epub 2022 Sep 3. Andrologia. 2022. PMID: 36056817
-
Nna VU, Ujah GA, Suleiman JB, Mohamed M, Nwokocha C, Akpan TJ, Ekuma HC, Fubara VV, Kekung-Asu CB, Osim EE. Nna VU, et al. Toxicology. 2020 Aug;441:152528. doi: 10.1016/j.tox.2020.152528. Epub 2020 Jun 18. Toxicology. 2020. PMID: 32565124
-
Nayak G, Rao A, Mullick P, Mutalik S, Kalthur SG, Adiga SK, Kalthur G. Nayak G, et al. J Ethnopharmacol. 2020 Sep 15;259:112922. doi: 10.1016/j.jep.2020.112922. Epub 2020 May 15. J Ethnopharmacol. 2020. PMID: 32422360
-
Amelioration of heat stress-induced damage to testes and sperm quality.
Shahat AM, Rizzoto G, Kastelic JP. Shahat AM, et al. Theriogenology. 2020 Dec;158:84-96. doi: 10.1016/j.theriogenology.2020.08.034. Epub 2020 Sep 8. Theriogenology. 2020. PMID: 32947064 Review.
References
-
- World Health Organization . 2010. Developing sexual health programmes A framework for action.
-
- García-Díaz EC, Gómez-Quiroz LE, Arenas-Ríos E., Aragón-Martínez A., Ibarra-Arias JA, Retana-Márquez MDSI. Oxidative status in testis and epididymal sperm parameters after acute and chronic stress by cold-water immersion in the adult rat. Syst Biol Reprod Med. 2015;61:150–160. doi: 10.3109/19396368.2015.1008071. – DOI – PubMed
BLACK HAWK: Best lions mane supplement for teens
Read the original publication: